
:max_bytes(150000):strip_icc()/co-why-is-carbon-dioxide-bad-4864246_V2-4ea7c0936b5a4cd3b8d4f2b41ec02f63.png)
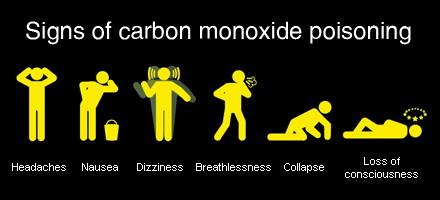
Brain damage and heart failure is an especially common side effect. While the human side effects of excessive carbon dioxide can be alleviated, carbon monoxide poisoning can easily lead to chronic complications, with heavy emphasis on the word chronic. This is much worse than what excessive carbon dioxide is capable of. When too much of this gas is present indoors, carbon monoxide poisoning occurs. Like carbon dioxide, you want to avoid taking in too much carbon monoxide. Lighting a match near it will create fatal consequences. It's capable of killing you faster than most other gases would.Īnd of course, it's extremely flammable too. It displaces oxygen from your blood causing air hunger, a feeling where you can’t take in enough air. It can severely damage your lungs, blood, and nervous system. Manufacturing companies will warn people, by law, to use those product outside, and for good reason too.Ĭarbon Monoxide is commonly known as “ the silent killer” due to it being a harmful gas that you technically can’t see or smell. Many appliances and products you can find in a supermarket are capable of producing carbon monoxide, like generators and charcoal grills.
This is why people immediately think of this gas when they see and smell those black smoke clouds from cars. While carbon monoxide has no color or odor, it can blend in with other burned gases. This means car fuel contains carbon monoxide. It also is used in industrial heating and fuel mixtures.
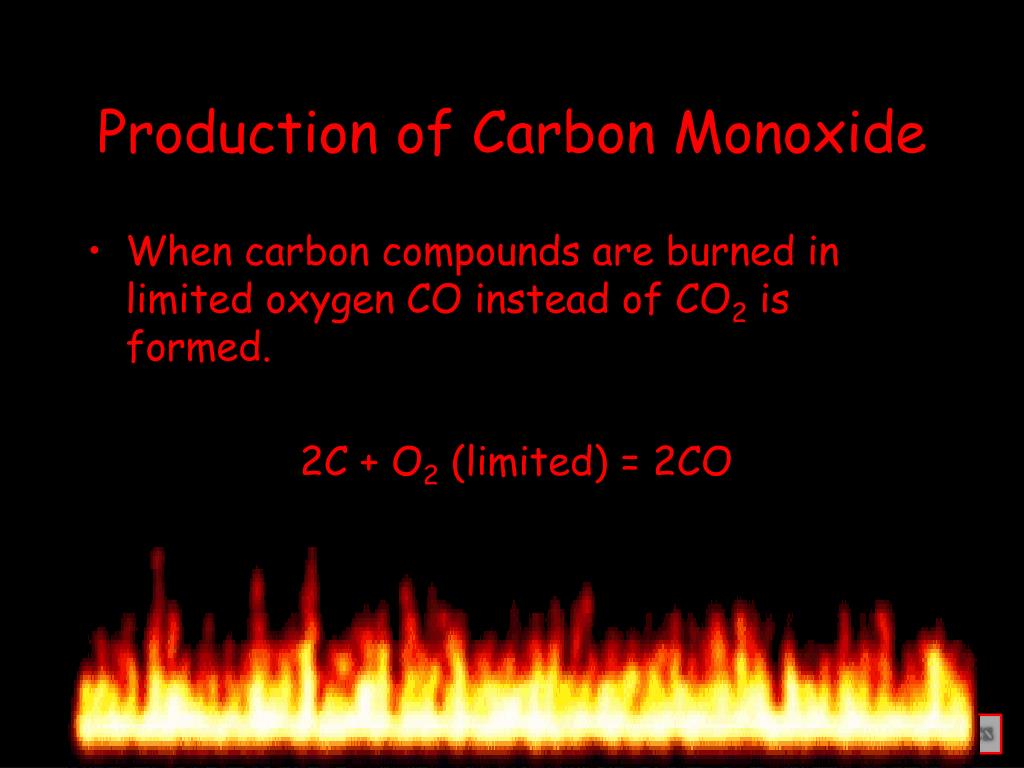
It reacts to specific metals like iron, cobalt, and nickel to form carbonyl compounds, which play a role in manufacturing plastic. It mostly serves as a means of manufacturing metals. Cars, gas ovens, and gas water heaters dispel CO for example, but they are typically safe as long as there's good ventilation.Ĭarbon monoxide doesn’t have any natural uses, only industrial applications. It results from badly ventilated fuel-burning appliances. It consists of only one oxygen atom and one carbon atom, hence its molecular formula: CO. The word monoxide comes from the Greek prefix “ mono,” meaning “ one”. So with that in mind, how does carbon monoxide compare?Ĭarbon monoxide is a colorless and odorless gas, just like carbon dioxide. It’s seen many times in the world of entertainment, and even has its place in the medical field. It keeps all living thing alive, including us, and even has a large variety of unique applications. Without it, life would be unsustainable.Ĭarbon dioxide is good for us. We create it every time we breathe, and it plays an important role in the carbon cycle, making photosynthesis possible. That’s why there is a “2” after the "O" in its molecular formula: CO2. It consists of one carbon compound and two oxygen compounds. The word “dioxide” comes from the Greek prefix “ di ”, which means “ two ”. It’s important to know the difference between them, as it could potentially save your life.Ĭarbon dioxide is a colorless and odorless gas.
They're both gases, but the two are completely different from each other. They kind of sound similar, don’t they? They both have " carbon", and they both have the same abbreviation, except one has a “2” at the end. Carbon dioxide (CO2) and carbon monoxide (CO).


 0 kommentar(er)
0 kommentar(er)
